Last Monday we conducted two experiments. The first was used to measure the relative heat of oil and water using the equation H = Cp*m*∆T, which determines the specific heat capacity, or how much energy is needed to raise the temperature of the substance one degree Celsius. The equation breaks down as this:
ΔE: Amount of energy absorbed (J)
m: mass of a liquid (g)
c: Heat capacity: (J/g-K)
ΔT : change in temperature (K)
r: mass density (g/ml)
Before the experiment we found the mass density of oil (.92) and water (1.0) as well as the specific heat of oil (2.0 J) and water (4.184 J).
For the actual experiment we added 80ml of oil and water to their own beakers and placed them on a hot plate, at which point we put in a temperature probe connected to the computer to record the data. Because of some issues with the hot plate where it was not heating as much as it should have the liquids did not change temperature as much as was needed, and according to the data, our water had a higher temperature than the cooking oil. Given the properties of oil and water and how oil heats up faster, we can only determine that this was an error.
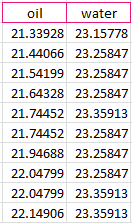
After the data was recorded, we calculated the difference of temperature from the start of the experiment to the end, and received a difference of .80978 degrees Celsius for the oil and .201135 degrees Celsius for the water.
We then calculated it further, which required the mass density and specific heat, and received an energy calculation of 67.39621 J for water and 119.1996 J for oil. The difference is 55.52473%, which demonstrates that oil needs more energy than water to be heated one degree. This however makes no sense considering that oil is less dense than water and therefore needs less energy to be heated, once again showing faulty data.
The second experiment focused on solar energy and what colors of light produce the most energy. For this we used a solar panel and sensor and a flashlight, as well as colored pieces of film that we held over the light. We initially experimented with the height of the flashlight to see how the distance between the sensor and the light affected the amount of energy produced. Predictably, with the height we determined that the further away the light was from the sensor, the less voltage was produced.
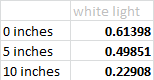
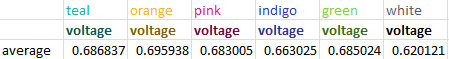
We used six colors total: teal, orange, pink, indigo, green and white (no filter) held at a height of zero inches from the sensor. By averaging the voltage produced we determined that orange had the highest amount of energy, while unfiltered light had the least.
Thank you for every other informative web site. The place else may I am getting that type of information written in such a perfect approach? I have a challenge that I’m simply now operating on, and I’ve been on the glance out for such information.
The variety of enemies in some games keeps combat interesting. https://vneconomictimes.com/
Hello.This post was really fascinating, especially because I was investigating for thoughts on this issue last Saturday.
I actually like reading by means of and I believe this site got some genuinely utilitarian stuff on it! .
The cooperative gameplay is super engaging. It’s awesome teaming up with friends. https://www.saferglasgow.com/crypto-casinos-uk/
Thanks a lot for giving everyone an extremely special chance to read critical reviews from here. It’s always very lovely and also stuffed with a lot of fun for me and my office mates to search the blog at least three times in one week to study the latest tips you will have. And of course, I am just actually contented for the outstanding information you serve. Selected 4 areas on this page are really the most suitable I’ve ever had.
Thank you that is very beneficial for me, as a new internet site has been inundated with comments that appear OK at 1st glance but then get repeated with a slight change of wording. I have something concrete to go on now and will delete quite plenty of them.
I respect your piece of work, appreciate it for all of the interesting content .
It’s actually a cool and useful piece of information. I’m happy that you simply shared this helpful information with us. Please keep us informed like this. Thanks for sharing.|
I would like to thnkx for the efforts you have put in writing this site. I’m hoping the same high-grade web site post from you in the upcoming as well. In fact your creative writing abilities has encouraged me to get my own web site now. Actually the blogging is spreading its wings fast. Your write up is a good example of it.
If I were the one having to write this content, all these readers would be disappointed. It’s a good thing you’re the writer and you bring fresh tips to us all. This really is fascinating.
Hi colleagues, its wonderful piece of writing concerning cultureand fully defined, keep it up all the time.|
I dont typically comment on blogs but i have to tell you nicely done
Hey mate, .This was an superb post for such a hard topic to speak about. I look forward to seeing numerous more outstanding posts like this one. Thanks
his could be the outstanding blog page for anyone who wants to know about this theme. You recognize a whole lot its virtually difficult to argue with you (not that I actually would want…HaHa). You completely put a fresh spin on a subject matter thats been published about for a lot of years. Wonderful issues, just superb!
Offers better conversions and traffic quality than Google.
Some truly fantastic info , Gladiolus I discovered this. “Speak when you are angry–and you will make the best speech you’ll ever regret.” by Laurence J. Peter.
I like what you guys are up too. Such intelligent work and reporting! Carry on the superb works guys I have incorporated you guys to my blogroll. I think it will improve the value of my website :).
I really enjoy examining on this internet site , it has good articles . “Something unpredictable but in the end it’s right, I hope you have the time of your life.” by Greenday.
The competitive mode in this game keeps me on my toes. Sòng bạc tạiCasino Truc Tuyen Online