The purpose of this blog is to educate and inform the reader of the experiment conducted by my group mates and myself. To do this I will discuss what the experiment is, what materials are needed for it, the procedure of the experiment, and some of the results we received when finished.
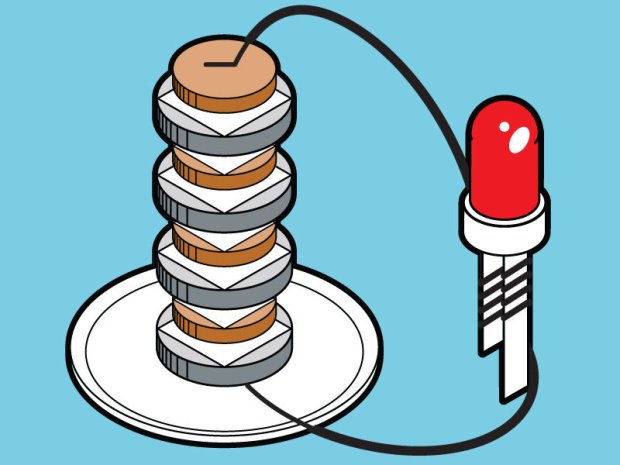
After a group brainstorm meeting at the end of a class session we decided that we wanted our experiment to be creating our own battery using everyday currency coins (pennies/nickels). In our experiment, we aim to create electricity (voltage) from coins. This invention will not save you if you run out of battery on your iPhone, but it is a curious, simple, and fun experiment. Our goal will be to measure the voltage we produce, and thus, realize that if we vary the number of pennies and nickel coins, the voltage also will vary.
The way that this works is somewhat simple. Batteries convert the chemical energy of the two metals (electrodes) interacting with the acid on the mat board (electrolyte) into electrical energy. In this situation, the metal surface serves as the electrode and an electric current (movement of electrons from one metal to the other) is created when the wire connects both metal surfaces.
So now that you know a little more about how it works I’ll provide you with the necessary materials so you can try it yourself. To create around 1 volt of electricity you will need:
– 6 pennies
– 6 nickels
– 6 pieces of cut paper towels (smaller than a nickel)
– 1 glass of water (filled with 2 tablespoons of salt)
– 1 Multimeter (to measure voltage)
Next I will explain the procedure of the experiment. The first step is to pour two tablespoons of salt into a glass of water and try to keep it mixed with the water. The second step is to put in the pieces of paper towel that were cut to the size of the nickel into the water/salt mix. A little bit smaller cuts are helpful because if paper towels in between different coins touch, it can mess up the current. We do not want the pieces of salt paper touching each other. Moisten the pieces of paper with the salt, and put them on each nickel or penny (based on which quantity of each you are measuring). Step 3 occurs once you have each piece of salt moistened paper placed on the coin. You then must stack the coins. If you are only using pennies or only using nickels then just simply stack coins with the paper in between them. If you are conducting the experiment using both coins in one then they must be stacked in a particular order. The sequence should be:
– Nickel, Paper, Penny, Nickel, Paper, Penny etc… (nickel should be at the bottom)
The last step is time to test your battery with a Multimeter. The multimeter measures how much voltage is coming from the coin battery. (Please note, you can continue to add more coin sequences to the top of the other coins to get more voltage.)
When we completed the experiment our results were what one would predict. Using the materials required for one volt of electricity got us to that level. What was interesting was that the voltage was very short lasting. To get an accurate result, the multimeter must measure the voltage right after the battery is assembled. We believe that the voltage could be longer lasting if we use a different material to put in between the coins. The material would have to be thicker and absorb more of the salt water. Other sources that conducted this experiment showed the use of small cardboard cut outs. Overall we found that when the quantity of coins used increases, so does the level of voltage. Also, we found that strictly using pennies for the battery is less efficient than using nickels and pennies.


Great post, you have pointed out some fantastic points , I likewise think this s a very wonderful website